Compliance & Quality Manager - Regulatory Affairs (w/m/d) in Healthcare/Medical Engineering
Isansys Lifecare Europe GmbH, Potsdam, Germany
Full-time, Permanent
Tasks / Responsibilities
- Further development and maintenance of our QM system according to EN ISO 13485 in collaboration with the QM department in UK
- Implementing and managing a post-market surveillance process according to the new MDR EU 2017/745
- Implementation and further development of risk management according to EN ISO 14971
- Communication with sales partners, authorities and notified bodies about regulatory topics
- Regular trend analysis reporting on product quality and internal key performance indicators to support senior management in taking effective decision for quality improvements
- Safety-Management: Collect and assess notifications of risks associated with medical devices that have become known and coordinate the necessary measures.
- Responsible for the fulfilment of notification obligations in so far as they relate to medical device risks
- Creation & implementation of training courses for quality-relevant procedures & processes
- Initiation, support and processing of internal corrective and preventive actions (CAPA)
- Support for product approval, preparation of clinical evaluations according to MEDDEV 2.7/1 and MDR
- Carrying out staff training courses to make employees aware of the importance of quality management for the company and our products
- Creation and maintenance of quality-relevant documents
- Responsibility for quality assurance in the context of development projects
- Promoting awareness of our customers' needs and regulatory requirements throughout the organisation
Qualifications
- University or University of Applied Science Degree with a focus on (Medical) Engineering, Life Science, Medicine, Natural Science or an equivalent Qualification
- At least two years of Professional experience in regulatory affairs and quality management in a medical device company or comparable positions
- Familiar with international regulations and standards pertaining to medical devices; familiarity with EN ISO 13485, EN ISO 14971 and ability to interpret and work with regulations and standards
- Expertise in risk management processes as well as notification and measure coordination/handling
- Knowledge of data analysis and statistical techniques
- Very good command of German and English, both verbal and written
- Self-starter attitude, strong organizational skills, flexibility, commitment, and ability to meet deadlines
- Goal oriented team player with excellent communication and conflict resolution skills
- Experienced in working Microsoft office
We offer:
- Attractive remuneration package with variable bonus, based on a scorecard system and company social benefits
- Open communication culture, international working atmosphere and flat hierarchies
- Mobile Office Regulation
- Cooperation in a very innovative medium-sized company
- Scope for personal and professional development, for initiative and commitment
- Targeted and intensive training in the mentoring system
- A varied and exciting job in an internationally active company
- A creative and open working atmosphere and collegial cooperation at eye level
- Short communication channels with effective decision-making
- In the inner conurbation of the metropolis Berlin
- Individual external or internal further training opportunities
Contact:
You are interested? Get in touch with: Michael Heinlein, Email: contact@isansys.eu by having a CV on hand.
Isansys Lifecare Europe GmbH
Potsdamer Str. 12b
Teltow Karree
14513 Teltow
NO AGENCIES PLEASE

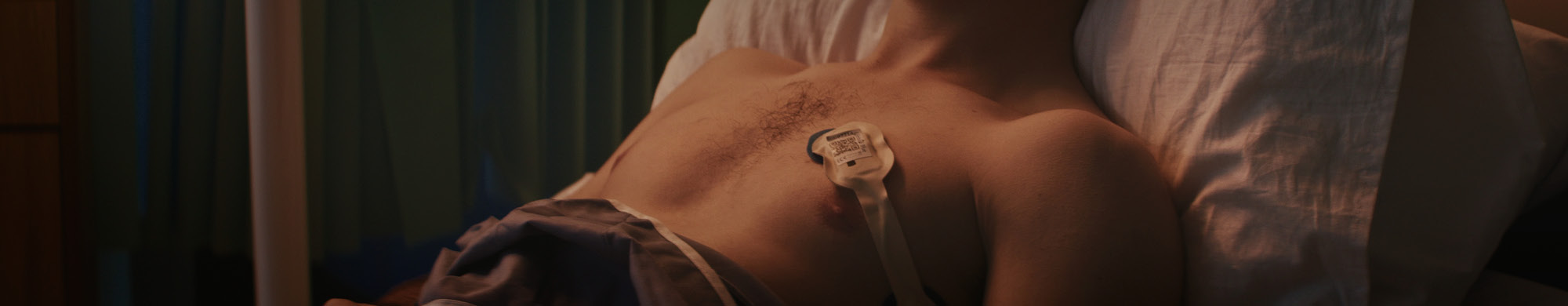
Isansys Lifecare Europe GmbH is a young digital health company that offers innovative, cost-effective and adaptable digital patient monitoring systems. Isansys Lifecare Europe GmbH was founded in Potsdam in April 2017 as a subsidiary of Isansys Lifecare Ltd. Thus, the worldwide leading company in future-oriented wireless monitoring is now also located in Germany. Together with worldwide users in hospitals, Isansys has developed a new generation of wireless vital data monitoring: The Patient Status Engine (PSE). This is an automated, wireless platform for telemonitoring. Using intelligent portable sensors, the patient's vital signs are collected and analysed. This is the first time that healthcare providers have access to continuous and forecast-supporting real-time data in hospitals, at home or in healthcare facilities. www.isansys.com
Our challenge: A progressively developing and sustainable maintaining quality management system tailored to our provision
Let us help you find what you’re looking for…